Solution :
Given : Volume = 5 L, temperature = 330 K
a). Moles in one litre,
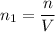
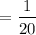
Number of atoms =

=
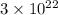
b). The thermal energy of the gas is :
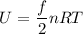
U = 10.16 L-atm
c). Given temperature, T = 380 K
Therefore, Δ S =
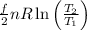
= 0.004 L-atm/K
d). When the expansion is negligible, the pressure of the heated gas is
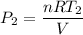
= 1.56 atm