Answer:
the required temperature of uranium hexafluoride is 54156.25 K
Step-by-step explanation:
Given the data in the question;
We know that average speed is;
u =
![[ 3RT / MM ]^{1/2](https://img.qammunity.org/2022/formulas/chemistry/high-school/ka973kmby9a36qkmv384dgjdhhc5yhl01f.png) ----------- let this be equation 1
----------- let this be equation 1
where MM is the molar mass
T is temperature
R is universal gas constant and u is the average speed.
First we get the average speed of H₂
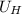 ₂ =
₂ =
![[ 3RT_H_2 / MM_H_2 ]^{1/2](https://img.qammunity.org/2022/formulas/chemistry/high-school/lvdj74gt04l4cjjutkn0ri71htvtvuzamf.png) ------ let this be equation 2
------ let this be equation 2
Next is the average speed of UF₆
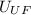 ₆ =
₆ =
![[ 3RT_(UF_6) / MM_(UF_6) ]^{1/2](https://img.qammunity.org/2022/formulas/chemistry/high-school/zvv5t9rmqsfwefkxndh81gi4w4ocgq6wkz.png) ------ let this be equation 3
------ let this be equation 3
given that; both have the same average speed, equation 2 = equation 3;
![[ 3RT_H_2 / MM_H_2 ]^{1/2](https://img.qammunity.org/2022/formulas/chemistry/high-school/lvdj74gt04l4cjjutkn0ri71htvtvuzamf.png) =
=
![[ 3RT_(UF_6) / MM_(UF_6) ]^{1/2](https://img.qammunity.org/2022/formulas/chemistry/high-school/zvv5t9rmqsfwefkxndh81gi4w4ocgq6wkz.png)
we multiply both sides by 1/3R and also square both sides.
![[ T_H_2 / MM_H_2 ]](https://img.qammunity.org/2022/formulas/chemistry/high-school/73at45bkv60efp83g6sd7i9dq024y56gqn.png) =
=
![[ T_(UF_6) / MM_(UF_6) ]](https://img.qammunity.org/2022/formulas/chemistry/high-school/eruvk3op7o7j7a62vbmttyoqkqibg7alyv.png)
given that; temperature of hydrogen T
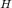 ₂ = 37°C = ( 37 + 273.15)K = 310.15 K
₂ = 37°C = ( 37 + 273.15)K = 310.15 K
we know that Molar mass of H₂; MM
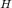 ₂ = 2.016 g/mol
₂ = 2.016 g/mol
and molar mass of UF₆; MM
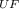 ₆ = 352.02 g/mol
₆ = 352.02 g/mol
so we substitute
[ 310.15 K / 2.016 g/mol ] = [ T
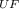 ₆ / 352.02 g/mol ]
₆ / 352.02 g/mol ]
T
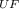 ₆ = [ 352.02 g/mol × 310.15 K ] / 2.016 g/mol
₆ = [ 352.02 g/mol × 310.15 K ] / 2.016 g/mol
T
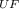 ₆ = 109179.003 K/ 2.016
₆ = 109179.003 K/ 2.016
T
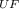 ₆ = 54156.25 K
₆ = 54156.25 K
Therefore, the required temperature of uranium hexafluoride is 54156.25 K