Answer:

Step-by-step explanation:
We are asked to convert 6.9*10²⁸ silver atoms to moles of silver. We can do this in 2 steps.
1. Convert Atoms to Moles
We know that 1 mole of any substance contains the same number of particles: 6.022*10²³ (Avogadro's Number). These particles can be atoms, molecules, formula units, and more. In this case, the particles are atoms of silver (Ag).
So, there are 6.022 *10²³ atoms of silver in 1 mole. Let's set up a ratio using this information.
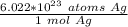
Since we are converting 6.9*10²⁸ silver atoms to moles of silver, we multiply by that value.
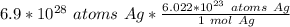
Flip the ratio. It remains equivalent, but the units if atoms of silver can cancel.
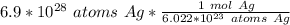

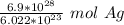
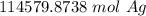
2. Round
The original measurement of silver atoms (6.9*10²⁸) has 2 significant figures, so our answer must have the same.
For the number we calculated, that is the ten thousands place. The 4 in the thousandths place (114579.8738) tells us to leave the 1.
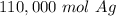
There are approximately 110,00 moles of silver in 6.9*10²⁸ silver atoms.