Answer:
m = 2.76 kg
Step-by-step explanation:
The mass of water evaporated can be found by using the following formula:
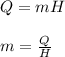
where,
m = mass of water evaporated = ?
Q = Energy used =
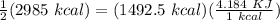 = 6244.62 KJ
= 6244.62 KJ
H = Latent heat of vaporization of water = 2260 KJ/kg
Therefore,
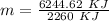
m = 2.76 kg