Answer: The stoichiometric coefficient for oxygen is
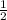 .
.
Step-by-step explanation:
A number present on the front of an atom, ion or molecule in a chemical reaction equation is called a stoichiometric coefficient.
For example,
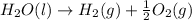
Here, the stoichiometric coefficient for
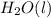 is 1, for
is 1, for
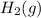 is 1 and for
is 1 and for
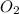 is
is
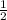 .
.
Thus, we can conclude that the stoichiometric coefficient for oxygen is
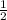 .
.