Answer:
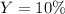
Step-by-step explanation:
Hello there!
In this case, according to the given information, whereas it is given both the theoretical and actual yield of copper metal, which are 86.04 g and 8.7 g respectively.
Next, since the percent yield is given by:
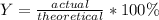
Next, we plug in the aforementioned yields to obtain:
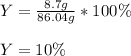
Regards!