Answer:
The answer is below
Step-by-step explanation:
The half life of a substance is the time required by that substance to reduce to half of its initial value. The half life is calculated using the formula:
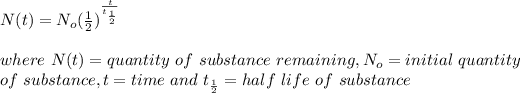
Given that t = 87 years, half life = 29 years, therefore the quantity of strontium-90 left is:
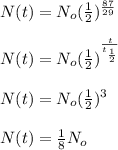
That is one-eight of Strontium 90 would be left after 87 years