Answer:
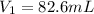
Step-by-step explanation:
Hello there!
In this case, according to this question, we will need to deal with this dilution problem, because it is asking for the volume of a 12.1-M stock solution of HCl. In such a way, we can use the following equation, under the assumption of no change in the number of moles in the solution:
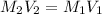
Thus, we solve for the initial volume, V1, as shown below:
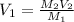
And plug in the initial concentration and final concentration and volume to obtain:
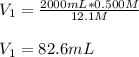
Regards!