Solution :
Molar mass of
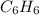 is :
is :
M = 6×12 + 6×1 g
M = 78 g
78 gram of
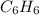 contains
contains
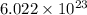 molecules.
molecules.
So, 89.5 gram of
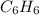 contains :
contains :
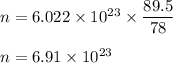
Now, from the formula we can see that one molecule of
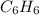 contains 2 hydrogen atom . So, number of hydrogen atom are :
contains 2 hydrogen atom . So, number of hydrogen atom are :
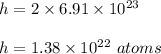
Hence, this is the required solution.