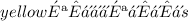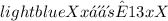Answer:
With an understanding of the ideal gas laws, it is now possible to apply these principles to chemical stoichiometry problems. For example, zinc metal and hydrochloric acid (hydrogen chloride dissolved in water) react to form zinc (II) chloride and hydrogen gas according to the equation shown below:
2 HCl (aq) + Zn (s) → ZnCl2 (aq) + H2 (g)
Step-by-step explanation: