Answer:
The gases in a hair spray can are at a temperature of 26.0 °C and a pressure of 25.0 lbs/in2.
If the pressure becomes
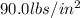 , what is the temperature of the gases?
, what is the temperature of the gases?
Step-by-step explanation:
According to Gay lussac's law:
the pressure of a gas is directly proportional to its absolute temperature.
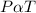
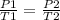
Given,
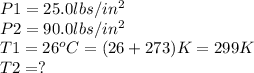
Substitute these values in the above formula:

Answer:
The gases will be raised to a temperature of 803.4
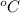 .
.