Answer:
V = 0.0327 L.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the liters of C3H6O by the definition of density. We can tell the density of this substance as that of acetone (0.784 g/mL) and therefore calculate the liters as shown below:
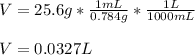
Regards!