Answer:
32 cm³
Step-by-step explanation:
The given gas data are;
The relative density of oxygen = 16
The relative density of carbon dioxide = 12
The time it takes 25 cm³ of carbon dioxide to effuse out = 75 seconds'
The duration of effusion of the oxygen = 96 seconds
The rate of effusion of carbon dioxide, R1 = 25 cm³/(75 sec) = (1/3) cm³/sec
According to Graham's law of diffusion and effusion of a gas, we have;
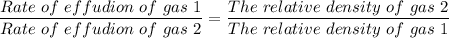
Therefore, we have;
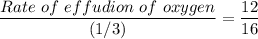
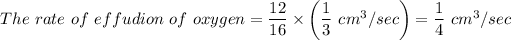
The volume of effusion = The rate of effusion × Time
The volume of the oxygen that will effuse in 96 seconds is given as follows;
The rate of effusion of a gas × Time
V = The rate of effusion of oxygen × Time = (1/3) cm³/sec × 96 sec = 32 cm³
The volume of oxygen that will effuse in 96 seconds, V = 32 cm³.