Answer:
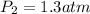
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to model the solubility and pressure of the gas, by knowing that the greater the pressure, the greater the solubility, so that the suitable equation is:
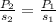
In such a way, we will be able to calculate the pressure of the gas by solving for P2 as shown below:
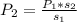
And finally plug in the given data to obtain:
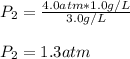
Regards!