Answer:

Step-by-step explanation:
We can convert particles to moles in 3 steps:
1. Convert Particles to Moles
First, we must convert particles to moles. 1 mole of any substance contains the same number of particles: 6.022*10²³ or Avogadro's Number. For this problem, we have particles of carbon dioxide or CO₂. We can make a ratio.
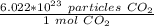
Since we are converting 1.5*10²² particles to moles, we multiply the ratio by that value.
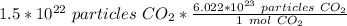
Flip the ratio. It remains equivalent, but the units of particles of carbon dioxide can cancel.
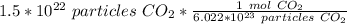
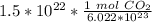
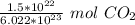
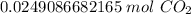
2. Convert Moles to Liters
There are 22.4 liters in 1 mole of any gas. Let's set up another ratio.
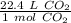
Multiply by the number of moles we calculated.
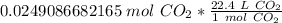
The units of moles of carbon dioxide cancel.
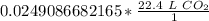
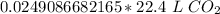
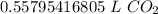
3. Round
The original measurement of particles (1.5*10²²) has 2 significant figures (1 and 5). Our answer must have the same number. For the number we calculated, that is the hundredth place.
The 7 in the thousandth place (0.55795416805) tells us to round the 5 in the hundredth place up to a 6.
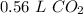
1.5*10²² particles of carbon dioxide is equal to 0.56 liters of carbon dioxide.