Answer:
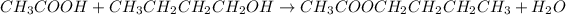
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to realize this is a question about esterification, process whereby a carboxylic acid and an alcohol react to produce water an an ester, we can set it up as shown below:
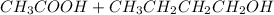
For the reaction between ethanoic acid and butanol; and therefore, the products side is:
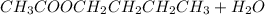
It means that the overall equation is:
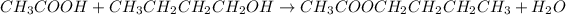
And the ester product is butyl ethanoate.
Regards!