Answer:
Step-by-step explanation:
First of all, I used the specific heat of water as 4182 J/(kgC) and the specific heat of ethyl alcohol (EtOH) as 2440 J/(kgC); that means that we need the masses in kg, not g.
120.g = .1200 kg of ethyl alcohol. Now for the formula:
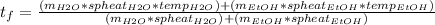 where spheat is specific heat.
where spheat is specific heat.
Filling that horrifying-looking formula in with some values:
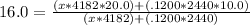 and
and
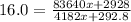 and
and
16(4182x + 292.8) = 83640x + 2928 and
66912x + 4684.8 = 83640x + 2928 and
1756.8 = 16728x so
x = .105 kg and the amount of water added is 105 g