Answer: The concentration of nitrogen gas in parts per million is 7.99 ppm.
Step-by-step explanation:
Solute is defined as the component of the solution present in a smaller proportion while the solvent is defined as the component of the solution present in a larger proportion.
Concentration in ppm (parts per million) represents the amount of solute in milligrams present per kilograms of solution.
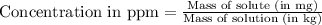
OR
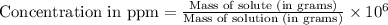 ......(1)
......(1)
Given values:
Mass of solute (nitrogen gas) = 0.008 g
Mass of solvent (water) = 1000 g
Mass of solution = [0.008 + 1000] g = 1000.008 g
Plugging values in equation 1:
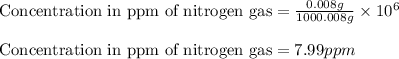
Hence, the concentration of nitrogen gas in parts per million is 7.99 ppm.