Answer: The correct answer is No solution.
Step-by-step explanation:
We are given:
Given mass of thiophene for the experiment = 90.00 g
12.3% w/w solution of thiophene
This means that 12.3 g of thiophene is present in 100 g of solution
Applying unitary method:
If 12.3 g of thiophene is present in 100 g of solution
So, 90.00 g of thiophene will be present in
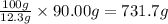 of solution
of solution
Converting it into kilograms:
1 kg = 1000 g
So, 731.7 g = 0.7317 kg
As the given amount of solution is 0.50 kg which is less than the required amount.
Thus, there is not enough solution the student should use.