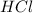Answer: Electrons are taken up by
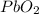 and they are lost by
and they are lost by
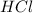
Step-by-step explanation:
Redox reaction is defined as the reaction in which oxidation and reduction take place simultaneously. It is also called the reaction where the exchange of electrons takes place.
An oxidation reaction is defined as the reaction in which a chemical species loses electrons takes place. In this reaction, the oxidation state of a substance gets increased.
A reduction reaction is defined as the reaction in which a chemical species gains electrons takes place. In this reaction, the oxidation state of a substance gets reduced.
For the given chemical reaction:
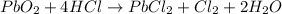
The half-reactions for this redox rection follows:
Oxidation half-reaction:
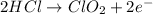
Reduction half-reaction:
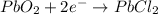
Hence, electrons are taken up by
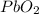 and they are lost by
and they are lost by