Answer:
The mole fraction of NaOH in an aqueous solution that contain 22.9% NaOH by mass=0.882
Step-by-step explanation:
We are given that
Aqueous solution that contains 22.9% NaOH by mass means
22.9 g NaOH in 100 g solution.
Mass of NaOH(WB)=22.9 g
Mass of water =100-22.9=77.1
Na=23
O=16
H=1.01
Molar mass of NaOH(MB)=23+16+1.01=40.01
Number of moles =
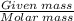
Using the formula
Number of moles of NaOH
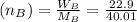
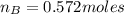
Molar mass of water=16+2(1.01)=18.02g
Number of moles of water
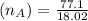
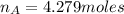
Now, mole fraction of NaOH
=
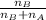
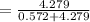
=0.882
Hence, the mole fraction of NaOH in an aqueous solution that contain 22.9% NaOH by mass=0.882