Answer:
Explanation:
The formula for this is
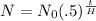 where
where
N is the amount of the element left after a certain amount of time (for us it is measured in days),
N₀ is the initial amount of the element in grams,
t is the number of days gone by, and
H is the half life of the element. Filling in:
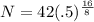 which simplifies a bit to
which simplifies a bit to
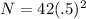 and a bit more to
and a bit more to
N = 42(.25) which gives us a remaining amount of
N = 10.5