Answer:
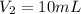
Step-by-step explanation:
Hello there!
In this case, according to the given information, it will be possible for us to solve this problem by using the Boyle's law as an inversely proportional relationship between pressure and volume:
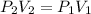
In such a way, we solve for the final volume, V2, and plug in the initial volume and pressure and final pressure to obtain:
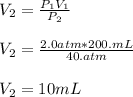
Regards!