Answer:
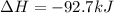
Step-by-step explanation:
Hello there!
In this case, according to the given information, we can infer that 890 kJ of energy are released when 1 mole of methane is burned; however, to find the total heat when 1.67 grams are burned, we first need to calculate the moles in this mass of methane:
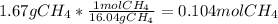
And thus, for calculating the resulting ∆H, we proceed as follows:
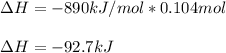
Regards!