Answer:

Step-by-step explanation:
In a chemical reaction, there are reactants and products.
The reactants are the substances at the beginning of the reaction. They go into the reaction and are changed.
The products are the substances created in the reaction and what you end up with after the reaction.
In an equation, the reactants are to the left of the arrow and the products are to the right.
Let's look at the equation given:
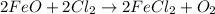
FeO and Cl₂ start the reaction and are to the left of the arrow, so they must be the reactants.