Answer:

Step-by-step explanation:
We are concerned with the variables of temperature and pressure, so we use Gay-Lussac's Law, which states the temperature of a gas is directly proportional to the pressure. The formula is:
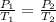
We know that the container of gas begins at standard temperature and pressure (STP). This is 1 atmosphere of pressure and 273 Kelvin.
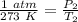
We know the gas is put into an oven at 280 degrees Celsius. We can convert this to Kelvin.
- K= °C + 273.15
- K= 280 +273.15
- K= 553.15
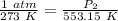
We are solving for the new pressure, so we must isolate the variable P₂. It is being divided by 553.15 Kelvin. The inverse of division is multiplication, so we multiply both sides by 553.15 K
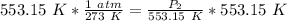
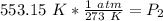
The units of Kelvin cancel.
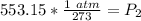
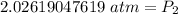
Rounded to the nearest hundredth:
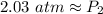
The new pressure is approximately 2.03 atmospheres.