The correct question is as follows: 0.500 moles of potassium oxide is dissolved in enough water to make 2.00 L of solution. Calculate the molarity of this solution (plz help!)
Answer: The molarity of this solution is 0.25 M.
Step-by-step explanation:
Molarity is the number of moles of a substance divided by volume in liter.
As it is given that there are 0.5 moles of potassium oxide in 2.00 L of water so, the molarity of this solution is calculated as follows.
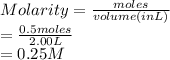
Thus, we can conclude that molarity of this solution is 0.25 M.