Answer:
The answer is "62.33 mol atoms".
Step-by-step explanation:
In the propane molecule, it includes 8 atoms of Hydrogen for every 3 atoms of Carbon or 8 mol Hydrogen atoms for each of the 3 mol Carbon atoms.
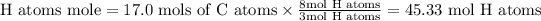
Total atoms = 17.0 mols of C atoms +45.33 mols of H atoms
=62.33 mol atoms