Answer:
m = 4.58 g.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to realize this problem is solved via the ideal gas equation:

As we can calculate the moles of propane given the pressure, temperature and volume as shown below:

Finally, since the molar mass of propane is 44.1 g/mol, we calculate the mass by following the shown below mole-mass conversion factor:
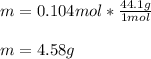
Regards!