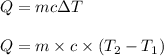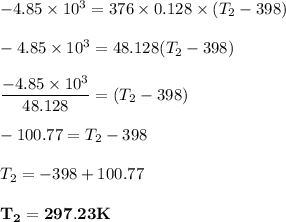Answer:
297.23 K
Step-by-step explanation:
Given that:
the mass of the gold nugget (m) = 376 g
the initial temperature
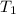 = 398 K
= 398 K
amount of heat lost Q = -4.85 kJ = -4.85 × 10³
specific heat capacity (c) = 0.128 J/g° C
Using the formula for calculating the Heat energy