Answer:
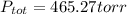
Step-by-step explanation:
Hello there!
In this case, according to the given information, it will be possible for us to use the Dalton's law, in order to solve this problem. However, we first need to calculate the mole fraction of oxygen by firstly calculating the moles of each gas:
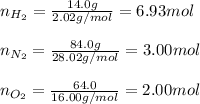
Next, we calculate such mole fraction as follows:
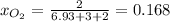
Then, given the following equation:
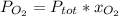
So we solve for the total pressure as follows:
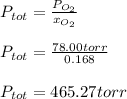
Regards!