Answer:
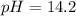
Step-by-step explanation:
Hello there!
In this case, according to the information in this problem, and considering these two bases are strong, it is necessary for us to calculate the total moles of OH ions as shown below:
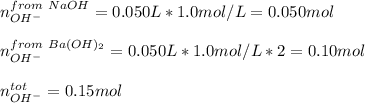
Now, the as the solutions are additive, the total volume is then 0.100 L and the concentration:
![[OH^-]=(0.15mol)/(0.100L)=1.5](https://img.qammunity.org/2022/formulas/chemistry/high-school/n7likztoczngx3yf5kdpxm1b6pnirlcm5x.png)
And therefore, the pH is:
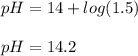
Regards!