Answer: 0.86 mol
This is a molarity problem, in which M is defined by:
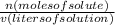 and the units are moles/liter
and the units are moles/liter
With this information in mind, we can plug in M and v already so that:
1.6
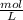 =
=
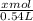 of Fe₂O₃
of Fe₂O₃
If we multiply 0.54 L from both sides, we will get:
0.864 mol = x mol of Fe₂O₃
Rounding that to sigfigs, we get that the number of moles within this Fe₂O₃ solution is 0.86 mol; therefore, that is the answer.
I hope this helped!