Answer:
There are 0.0305 calories in 0.128 joules
Step-by-step explanation:
Given that,
Heat absorbed, Q = 0.128 J
We need to find the heat energy absorbed in calories.
We know that the relation between joules and calories is as follows :
1 calorie = 4.184 J
1 J = (1/4.184) J
So,
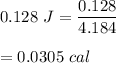
So, there are 0.0305 calories in 0.128 joules