Answer:
The pressure value changes 400 % relative to the initial value.
Step-by-step explanation:
Let suppose that the gas behaves ideally and represents a closed system, that is, a system with no mass interactions so that number of moles is conserved (
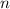 ). Since the variables involved in the isothermal process are pressure (
). Since the variables involved in the isothermal process are pressure (
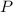 ) and volume (
) and volume (
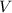 ). Finally, the process is represented by the following relationship:
). Finally, the process is represented by the following relationship:
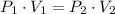 (1)
(1)
Where:
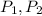 - Initial and final pressures.
- Initial and final pressures.
 - Initial and final volumes.
- Initial and final volumes.
If we know that
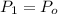 ,
,
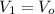 and
and
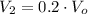 , then the final pressure of the closed system is:
, then the final pressure of the closed system is:
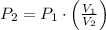
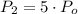
The pressure value changes 400 % relative to the initial value.