Answer:
1. 2.510kJ
2. Q = 1.5 kJ
Step-by-step explanation:
Hello there!
In this case, according to the given information for this calorimetry problem, we can proceed as follows:
1. Here, we consider the following equivalence statement for converting from calories to joules and from joules to kilojoules:
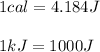
Then, we perform the conversion as follows:
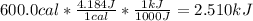
2. Here, we use the general heat equation:
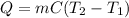
And we plug in the given mass, specific heat and initial and final temperature to obtain:
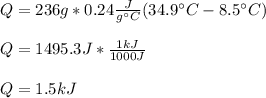
Regards!