Answer:
b) -25 kJ/mol.
Step-by-step explanation:
Hello there!
In this case, since the general definition of the enthalpy of reaction involves the subtraction of the energy of the products and the energy of the reactants:
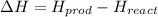
Thus, since the graph shows that the energy of the products is 20 kJ/mol and that of reactants 45 kJ/mol, we will obtain:
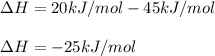
Which means it is exothermic.
Regards!