Answer:
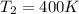
Step-by-step explanation:
Hello there!
In this case, according to the given information for this question, it turns out possible for us to calculate the final temperature, after the expansion process, by using the well-known Charles' gas law:
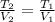
Thus, we solve for the final temperature to obtain:
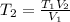
And plug in the given values:
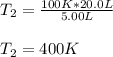
Regards!