Answer:
Step-by-step explanation:
We want to convert from moles to grams, so we must use the molar mass.
1. Molar Mass
The molar mass is the mass of 1 mole of a substance. It is the same as the atomic masses on the Periodic Table, but the units are grams per mole (g/mol) instead of atomic mass units (amu).
We are given the compound PI₃ or phosphorus triiodide. Look up the molar masses of the individual elements.
- Phosphorus (P): 30.973762 g/mol
- Iodine (I): 126.9045 g/mol
Note that there is a subscript of 3 after the I in the formula. This means there are 3 moles of iodine in 1 mole of the compound PI₃. We should multiply iodine's molar mass by 3, then add phosphorus's molar mass.
- I₃: 126.9045 * 3=380.7135 g/mol
- PI₃: 30.973762 + 380.7135 = 411.687262 g/mol
2. Convert Moles to Grams
Use the molar mass as a ratio.
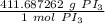
We want to convert 3.14 moles to grams, so we multiply by that value.
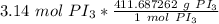
The units of moles of PI₃ cancel.
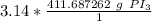
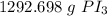
3. Round
The original measurement of moles has 3 significant figures, so our answer must have the same. For the number we calculated, that is the tens place.
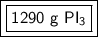
The 2 in the ones place tells us to leave the 9.
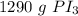
3.14 moles of phosphorous triiodide is approximately equal to 1290 grams of phosphorus triodide.