Answer:
the packing efficiency is 52.36%
Step-by-step explanation:
Given the data in the question;
simple cubic unit cell that contains one atom with a metallic radius of 175 pm;
we know that;
Edge length of Simple cubic (a) is related to radius of atom (r) as follows;
a = 2r
since radius r = 175 pm
we substitute
a = 2 × 175 pm
a = 350 pm
Now we get the volume unit;
Volume of unit cell = a³ = ( 350 pm ) = 42875000 pm³
Next we get Volume of sphere;
Volume of Sphere =
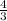 πr³
πr³
Volume occupied by 1 atom =
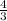 × π × ( 175 pm )³
× π × ( 175 pm )³
=
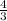 × π × 5359375 pm³
× π × 5359375 pm³
= 22449297.5 pm³
Now, the packing efficiency = ( Volume occupied by 1 atom / Volume of unit cell ) × 100
we substitute;
packing efficiency = ( 22449297.5 pm³ / 42875000 pm³ ) × 100
= 0.523598 × 100
= 52.36%
Therefore, the packing efficiency is 52.36%