Answer:
The answer is "
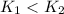 "
"
Step-by-step explanation:
In the given question, the value of the
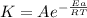 and the
and the
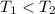 is the rate value which is the constant that is
is the rate value which is the constant that is
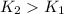 . As per the temperature value when its increase rate is constantly increasing.
. As per the temperature value when its increase rate is constantly increasing.
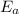 is activation energy it is not dependent on the temperature that why the answer is
is activation energy it is not dependent on the temperature that why the answer is
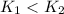 .
.