Answer:
Explanation:
We get to use the simple version of the half life equation:
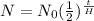 where N is the amount of radioactive element left after a specific number of years,
where N is the amount of radioactive element left after a specific number of years,
N0 is the initial amount of the element,
t is the number of years (our unknown), and
H is the Half life of the element. For us,
N is 61
N0 is 70,
t is unknown,
H is 24 years. Filling in:
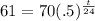 . We begin by dividing both sides by 70 to get:
. We begin by dividing both sides by 70 to get:
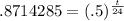 and then take the natural log of both sides:
and then take the natural log of both sides:
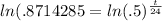 which allows us to bring down the exponent to the front on the right side:
which allows us to bring down the exponent to the front on the right side:
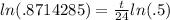 . We divide both sides by ln(.5) to get:
. We divide both sides by ln(.5) to get:
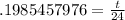 and then multiply both sides by 24 to get:
and then multiply both sides by 24 to get:
t = 4.8 years