Answer:
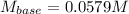
Step-by-step explanation:
Hello there!
In this case, according to the given information in the undergoing chemical reaction, it is possible for us to realize that barium hydroxide reacts with hydrochloric acid in a 1:2 mole ratio, which means that at the equivalence point, we have:
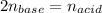
That can be written in terms of molarity and volume:
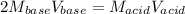
Thus, we solve for the molarity of the barium hydroxide to obtain:
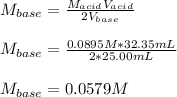
Regards!