Answer:
The mole fraction of N₂ is 0.26.
Step-by-step explanation:
The pressure exerted by a particular gas in a mixture is known as its partial pressure. So, Dalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
PT = PA + PB
This relationship is due to the assumption that there are no attractive forces between the gases.
Dalton's partial pressure law can also be expressed in terms of the mole fraction of the gas in the mixture. The mole fraction is a dimensionless quantity that expresses the ratio of the number of moles of a component to the number of moles of all the components present.
So in a mixture of two or more gases, the partial pressure of gas A can be expressed as:
PA = XA * PT
In this case:
- PA= PN₂= 300 torr
- XA=XN₂= ?
- PT= 1.50 atm= 1140 torr (being 1 atm= 760 torr)
Replacing:
300 torr= XN₂*1140 torr
Solving:
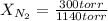
XN₂= 0.26
The mole fraction of N₂ is 0.26.