Answer:
By taking 18.8 mL of the stock solution and adding about 781.2 mL of water to obtain the desired dilution
Step-by-step explanation:
Hello there!
In this case, for this dilution problem, it will be possible for us to calculate required volume of the stock NaOH solution by considering that the moles of this solute remain unchanged so we can use the following equation:
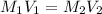
Whereas the subscript 1 stands for the stock solution and 2 for the dilution. In such a way, we solve for the volume of the former, V1, as shown below:
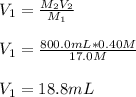
It means we take 18.8 mL of the stock solution and add about 781.2 mL of water to obtain the desired dilution.
Regards!