Answer: The partial pressure of
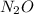 is 0.35 atm.
is 0.35 atm.
Step-by-step explanation:
Given: Total pressure = 0.98 atm
Partial pressure of
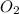 = 0.48 atm
= 0.48 atm
Partial pressure of Ar = 0.15 atm
Partial pressure of
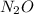 = ?
= ?
Total pressure is the sum of partial pressure of each component present in a mixture of gases.
Hence, partial pressure of
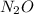 is calculated as follows.
is calculated as follows.
Total pressure =
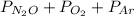
Substitute the values into above formula as follows.
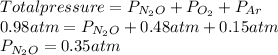
Thus, we can conclude that the partial pressure of
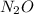 is 0.35 atm.
is 0.35 atm.