Answer:
The value of K is 0.027.
Step-by-step explanation:
Chemical equilibrium is a state of a reversible chemical system in which no changes are observed as time passes, despite the fact that the substances present continue to react with each other. That is, the chemical equilibrium is established when there are two opposite reactions that take place simultaneously at the same speed.
The Law of Mass Action says that the product of the equilibrium concentrations of the products raised to their respective stoichiometric coefficients, divided by the product of the concentrations of the reactants in the equilibrium raised to their respective stoichiometric coefficients, is a constant for each temperature, called the equilibrium constant Kc. So, being:
aA + bB ⇄ cC + dD
Then:
![Kc=([C]^(c) *[D]^(d) )/([A]^(a) *[B]^(b) )](https://img.qammunity.org/2022/formulas/chemistry/high-school/bqtwr85tvvfqy5lkvl057xxprvr50i38mv.png)
In this case, the reaction is:
N₂ + 3 H₂ ⇒ 2 NH₃
So, the equilibrium constant is:
![Kc=([NH_(3) ]^(2) )/([N_(2) ]*[H_(2) ]^(3) )](https://img.qammunity.org/2022/formulas/chemistry/high-school/ih50o1yfuq4aiz67uvhez8ttgkdq93cw97.png)
Being:
- [N₂] = 9.76
- [H₂] = 6.52
- [NH₃]= 8.62
Replacing:
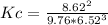
Solving
Kc= 0.027
The value of K is 0.027.