Answer:
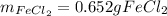
Step-by-step explanation:
Hello there!
In this case, according to the given information and the chemical reaction, whereby iron and hydrochloric acid react in a 1:2 mole ratio, it is firstly necessary to calculate the moles of iron (II) chloride from each reactant in order to figure out the limiting reactant:
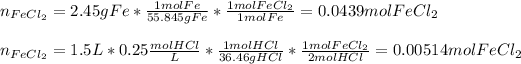
In such a way, we infer the maximum moles of FeCl2 product are yielded by HCl, for which it is the limiting reactant. Finally, we calculate the grams of product by using its molar mass as shown below:
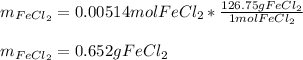
Regards!