Answer:
The answer is "208 mL".
Step-by-step explanation:
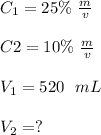
In dilutions of different concentration, its initial volume for glucose solution V1 can be obtained by using the following formula, i.e.
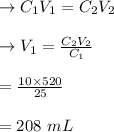
The solution of 520 mL
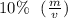 final glucose solution thus requires 208 mL of glucose solution of
final glucose solution thus requires 208 mL of glucose solution of
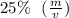 .
.