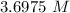Answer:
The correct solution is "3.6975 M".
Step-by-step explanation:
The given values are:
Volume of solution,
V = 23.2 mL
Density,
D = 1.42 g/mL
Final volume,
= 200 mL
or,
= 0.2 L
Now,
The mass will be:
=
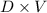
=

=
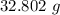
Mass of HNO₃ will be:
=
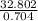
=
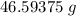
Mol of HNO₃ will be:
=
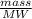
=
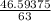
=
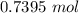
hence,
The concentration will be:
=
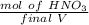
=
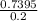
=